Aim: Drug development programs in rare diseases face many challenges, such as the scarcity and geographic dispersion of patients, limited natural history data, the need for novel study designs, and sensitive outcome measures.
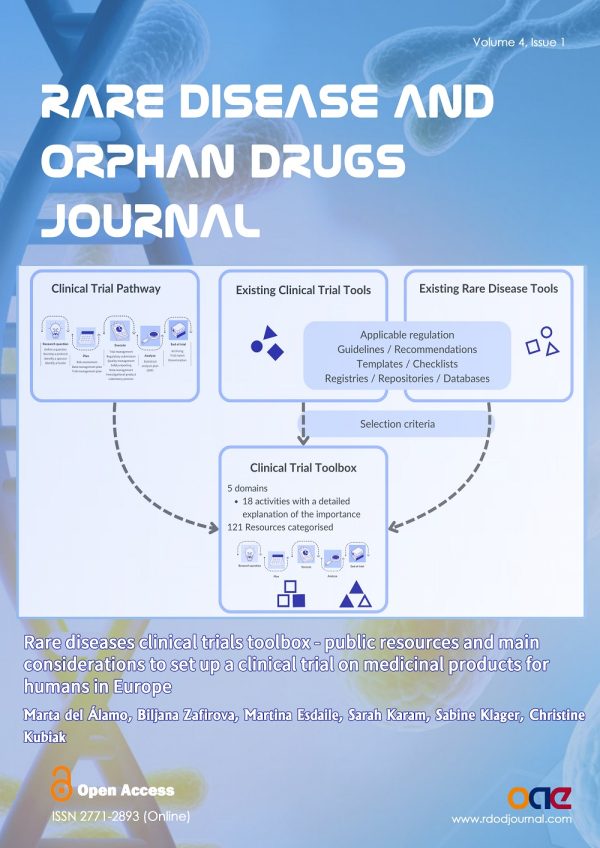
Over the past years, tools supporting clinical research have been developed in the context of different projects and for diverse purposes. Therefore, they have not yet been structured to encompass the conduct of rare disease (RD) clinical trials as a whole. To address this issue, the European Joint Program for Rare Diseases (EJP RD) has developed the Rare Diseases Clinical Trial Toolbox. This toolbox collates the accumulated knowledge (collectively termed “tools”) generated by projects/organizations into a structured and guided instrument. By structuring and making resources discoverable, we aim to help RD clinical trialists and trial managers.
Methods: We have designed and developed a toolbox structured around the definition of the clinical trial pathway. It is organized into five domains. Each domain describes one or several activities to be considered throughout the clinical trial pathway. Selection criteria were then defined to include existing resources that are relevant to those activities. Rare disease-specific resources are highlighted as such and include those especially relevant to pediatric clinical research.
Results: The current version of the Toolbox includes 121 resources tagged as relevant to any of the 18 activities within the clinical trial pathway. Overall, 60% of all resources are relevant to any clinical trial while 40% are tagged as “rare disease-specific”.
Conclusion: Access to public resources relevant to the development of clinical trials for rare diseases is sometimes challenged by limited awareness and/or the absence of an adequate framework that enables their findability. This Toolbox aims to build a framework supporting the optimal use of existing tools.