
On 10 December 2025, ERDERA launched its 2026 Joint Transnational Call, “Resolving unsolved cases in rare genetic and non‑genetic diseases”.
The call welcomes proposals focused on providing diagnostic clarity in unsolved rare genetic and non‑genetic diseases.
An information webinar for potential applicants will take place on 16 December, 15:00–17:00 CET. Register for the webinar through this link.
Involving patient organisations from the very start is highly desirable. Should you need guidance about patient involvement in research, have a look at this guide.
01 · Aims & focus of the call
Topic List
The goal of this call is to solve Undiagnosed Rare Genetic diseases and to address complex,
multifactorial Rare Non-Genetic diseases by identifying causative variants in patients with no
molecular diagnosis after prior genetic or genomic testing and providing diagnostic clarity for
conditions of unknown or mixed pathogenesis.
Suggested focus areas are:
- Functional validation to classify variants of uncertain significance (VUS) and increase the
diversity of functional genomics research, or validation of candidate VUS to improve
outcomes for a broader range of patients using in silico, in vitro or animal model systems
(e.g. CRISPR modified cells, iPSCs, organoids, etc.); - Use of multi-omics or integrative methods (e.g. transcriptomics, epigenomics, etc.) to
resolve ambiguous or complex variants; - New tools/methodologies not yet validated in clinical setting, including biostatistics,
advanced bioinformatics, and mathematics approaches (e.g. variant effect predictors,
Artificial Intelligence (AI)-based annotation platforms, etc.); - Systems biology and disease mechanism modelling;
- Integration of clinical, environmental, lifestyle, and sensor-derived data;
- Development of knowledge graphs or disease maps to link phenotypic and mechanistic
insights; - Use of advanced AI and modelling tools (graph ML, probabilistic causal models).
Excluded Diseases, Approaches and Topics
The following diseases, approaches and topics are excluded from the scope of the JTC 2026:
- Pre-clinical therapy development studies as covered in ERDERA JTC2025 topic.
- Interventional clinical trials to prove efficacy of drugs/treatments/surgical procedures/medical
procedures. This includes studies comparing efficacy, e.g., two surgical techniques or therapies,
and projects whose main objective is the implementation of a clinical phase IV
pharmacovigilance study; - Projects focusing only on rare neurodegenerative diseases that are within the focus of Brain
Health Partnership. These are: Alzheimer’s disease and other dementias; Parkinson’s disease
(PD) and PD-related disorders; prion diseases; motor neuron diseases; Huntington’s disease;
spinal muscular atrophy and dominant forms of spinocerebellar ataxia. However, childhood
dementias/neurodegenerative diseases are eligible; - Rare infectious diseases, rare cancers and rare adverse drug events in treatments of common
diseases. Rare diseases with a predisposition to cancer are eligible. Diseases with inborn errors
of immunity/genetic predisposition to rare infectious disease are eligible.
02 · Consortium makeup
Limit number of partners
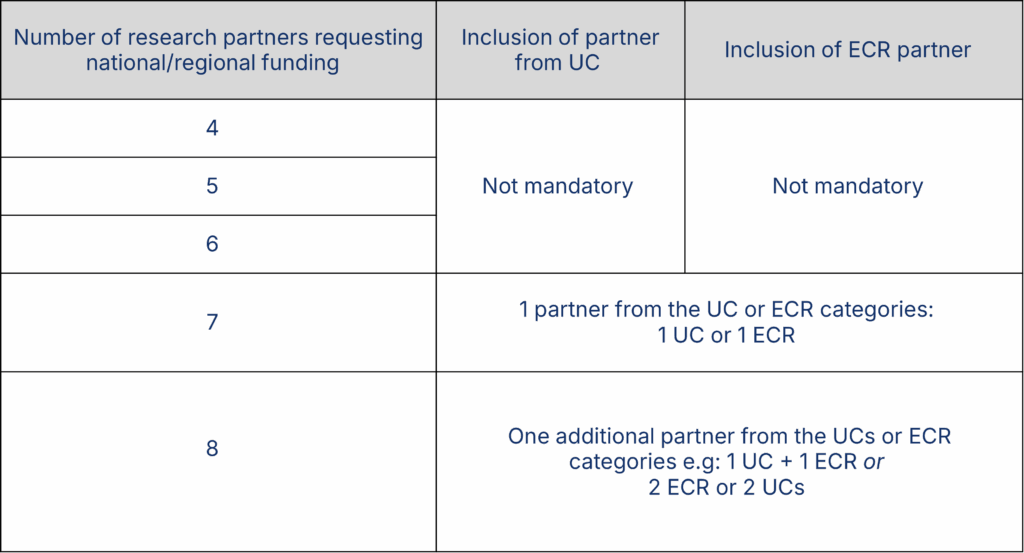
Only transnational projects will be funded. Each consortium submitting a proposal must involve four to six eligible principal investigator partners (referred to as partners below) from at least four different participating countries (see list in section 2). In specific cases, the number of consortium partners can be increased to eight partners (see table below). No more than two eligible partners from the same country can be present in each consortium; further national/regional limits may apply, see “Guidelines for Applicants”. Patient partners, requesting funding or not, do not count toward the total.
The number of partners can be increased to 8 in two cases:
- The inclusion of partners from participating usually underrepresented countries (UCs) in projects (UCs: Estonia, Latvia, Lithuania, Slovakia, Türkiye), OR
- The inclusion of an ECR as full partner (see section 5.6).
What is a partner? a collaborator? a sub-contractor?
To be considered as an eligible partner, a group must contribute substantially to at least one of the project’s work packages. If the only role of a group is to provide patient access, data or samples for the study, they will not be considered as partners of the consortium, but can be included otherwise, via cooperation agreements (as collaborators) or subcontracting.
Consortia may include collaborators who secure their own funding. Collaborators cannot be work package leaders, and their contribution to the consortium must be described. As they do not receive funding as part of this call, they do not count toward the limit of 8 partners requesting research funding (nor is there a limit of collaborators per country, if their participation is justified). Collaborators must supply a letter of intent, CV (only at the full proposal stage), which must be entered into the online submission system.
If necessary, to implement the research activity, consortia may also include subcontractors if allowed by their country/regional regulations. Sub-contractors may cover only a limited part of the research activity, and their contribution to the consortium must be described. They do not count toward the limit of 8 partners requesting research funding (nor is there a limit of subcontractors per country, if their participation is justified and if subcontracting is possible according to national/regional funding rules).
Patient Advocacy Organisations and Patients’ group list
Consortia should include and actively engage at least one patient partner, i.e. a patient representative from an organized group (preferably from a patient advocacy organisation (PAO)) from the start when preparing their proposals. For information on any PAOs or patients’ group dedicated to undiagnosed PLWRD, please see:
- NORD.
03 · Important dates
Call text with guidelines, aims of the scheme and applicants information. Call text accessible on ERDERA website
Information webinar for those who might be interested to apply
Complete online submission and upload completed application template including additional doccuments
Invitations for selected projects to proceed with a full proposal
Information webinar for applicants invited to submit a full proposal
Complete online submission and upload completed application template including additional doccuments
Formal notifications sent to all applicants. Public announcement on ERDERA website and social channels for selected applications
04 · A quick guide through the submission process
1/ Read carefully Call text and guidelines.
2/ Use the pre-proposal form to prepare the submission of the project online.
3/ Make sure the following points are checked before submission.
General conditions:
- The project proposal addresses the AIM/S of the call
- The project proposal meets the TOPIC/S included in this call
Ethical standards:
The proposal complies with ethical principles (including the highest standards of research integrity — as set out, for instance, in the European Code of Conduct for Research Integrity — and including, in particular, avoiding fabrication, falsification, plagiarism or other research misconduct).
Composition of the consortium:
The project proposal involves at least 4 eligible research partners from at least 4 different countries participating in the call.
The project proposal does not include more than two eligible research partners from the same partner country participating in the call (check out additional national limits that apply, in “Guidelines for Applicants”).
The consortium coordinator and the partners are eligible to receive funding from their national funding organisation(s) participating in the call.
The project proposal involves a maximum of 6 eligible research partners (including the coordinator) asking for funding. In case of inclusion of partners from participating underrepresented countries (Estonia, Latvia, Lithuania, Slovakia, Türkiye) or additional early career researchers, the project involves a maximum of 8 eligible partners asking for funding.
Eligibility of consortium partners:
I have checked that each research partner involved in the project proposal is eligible to receive funding by its funding agency.
I have checked that the applicants have confirmed the eligibility of the pre-proposal with their national/regional Contact Point.
I have checked that the mandatory early career researcher fulfills the eligibility criteria for an ECR.
(If applicable) Italian partners applying for funding at the Ministry of Health involved in the proposal have submitted a pre-submission eligibility check form to their national funding organization at least 10 working days before the submission deadline, through their IRCCS, using WorkFlowResearch System-> ER communication code. The pre-eligibility check form is available here: https://www.salute.gov.it/imgs/C_17_pagineAree_4441_0_file.pdf
(If applicable) Italian partners applying for funding at Tuscany Region involved in the proposal have submitted a pre-submission eligibility check form duly filled and signed by the Tuscan Principal Investigator and by the legal representative of the beneficiary to their regional funding organization (erdera@regione.toscana.it) at least 10 days before the submission deadline.
(If applicable) Italian partners applying for Fondazione Telethon should submit a pre-submission eligibility check form to Fondazione Telethon at least 10 working days before the submission deadline.
(If applicable) Austrian partners have submitted administrative and financial data (in accordance with the FWF guidelines for stand-alone projects) online to the FWF at https://elane.fwf.ac.at/.
(If applicable) Hungarian partners have submitted mandatory information to NKFIH, including applicant name and affiliation, as well as an estimation of the requested budget.
(If applicable) Slovak partners have submitted a Letter of Commitment of the partner institute’s in-kind contribution (spoluucast) to SAS.
(If applicable) Turkish partners will submit the pre-proposal within 2 weeks, including the electronic signature process, through TUBITAK UIDB application system: http://uidb-pbs.tubitak.gov.tr/.
(If applicable) Israeli partners have submitted to CSO-MOH an abstract and budget table and received approval for eligibility prior to the submission of the preproposal to ERDERA (in accordance with CSO-MOH guidelines)
(If applicable) Luxembourgish partners must submit the pre-proposal as well as INTER documents to the FNR up to 7 working days after the submission deadline (see https://www.fnr.lu/funding-instruments/inter/).
(If applicable) Portuguese partners need to send the Statement of Commitment to FCT (erdera@fct.pt) up to 10 working days after the deadline for submission of pre-proposals.
(If applicable) Cypriot partners through the Coordinator of the Cypriot Consortium should also submit a pre-proposal and full proposal to the RIF at (https://iris.research.org.cy) up to 7 calendar days after the submission deadline of pre-proposals and full proposals, respectively.
(If applicable) Czech partners have submitted to AZVCR a Sworn Statement, Sworn Statement of composition consortium, and Application form for national eligibility check no later than the deadline for submission of pre-proposals to ERDERA (https://www.azvcr.cz/).
(If applicable) Belgian partners applying for funding to F.R.S.-FNRS have checked that they are in accordance with the eligibility rules and criteria, which can be found in the PINT-Multi regulations. It is strongly advised to contact the F.R.S.-FNRS prior to submission regarding the eligibility criteria. Applicants to F.R.S.-FNRS funding must provide basic administrative data by submitting an administrative application on e-space within 5 working days after the general deadline of ERDERA call to be eligible. Please select the “PINT-MULTI” funding instrument when creating the administrative application.
(If applicable) (If applicable) Belgian partners applying for funding to SPW are requested to contact SPW at least 4 weeks before the submission deadline. Applicants have checked that they are in accordance with the eligibility rules and criteria which can be found in the SPW-Recherche website and the “Guidelines to applicants” edited by the partnership. Applicants to SPW funding must fill in the regional pre-proposal form on the regional application platform ONTIME. Proposals invited to the second stage must also be submitted on the same platform. The submission deadlines are the same as the general deadline of the ERDERA call.
(If applicable) Canadian partners will submit applicant information and the pre-proposal at the pre-proposal stage and the full proposal at the full proposal stage as per CIHR Funding Opportunity (link to follow).
