Current European landscape
The variation in screening programmes creates a concerning “postcode lottery” for newborns. A child born in one country may receive early diagnosis and life-changing treatment for a condition that would go undetected in a neighbouring nation until symptoms appear—often too late for optimal outcomes.
EU Council Recommendation on Rare Diseases first highlights screening disparities
European Reference Networks establish working groups on screening harmonization
ERDERA launched with €380 million funding under Horizon Europe
Implementation of coordinated screening expansion across member state
Key disparities
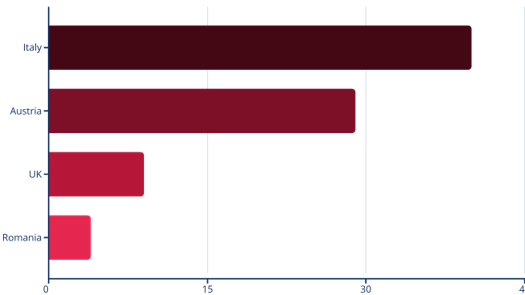
Number of conditions screened by country:
As shown in the chart, there are significant disparities in the number of conditions screened for newborns across European countries. Italy leads with 40 conditions, followed by Austria with 29. In stark contrast, the UK screens for only 9 conditions, and Romania for just 4, highlighting the “postcode lottery” that ERDERA aims to eliminate.
ERDERA strategic pillars
Data infrastructure
Creating shared European databases to evaluate screening outcomes, establish benchmarks, and validate new tests. This includes a federated data network linking biobanks, registries, and clinical databases while ensuring GDPR compliance.
Standardised protocols
Developing evidence-based guidelines for implementation that can be adapted to different healthcare systems, including cut-off values, confirmation protocols, and referral pathways for positive results.
Workforce development
Supporting training programs for laboratory personnel, clinicians, and genetic counsellors to ensure proper interpretation of advanced screening technologies and appropriate communication with families.
Collaborative governance
Facilitating dialogue between stakeholders—including patient representatives, healthcare providers, and policymakers—to guide evidence-based policy decisions and ethical implementation.
Economic impact
Sources: ERDERA Strategic Plan 2022-2027; European Commission Health Programme Evaluation 2023; International Journal of Technology Assessment in Health Care (2021); Rare2030 Foresight Study.




