Structure and Governance
Addressing rare diseases from multiple perspectives
ERDERA has been designed as a system comprising seven integrated services to move rare disease research forward.
Patient and public involvement and engagement (PPIE) is woven throughout, guiding all services, activities, and governance across the partnership.
Funding
ERDERA will provide financial support for collaborative international research projects, clinical trials, and knowledge exchange and networking initiatives.
Clinical Research
Encompassing all ERDERA’s in-house research activities, this area enhances diagnostics and clinical trial readiness, helps assess the impact of rare diseases and supports the development of advanced therapies.
It also builds on the clinical expertise of the 24 European Reference Networks (ERNs) established in 2017 thanks to the Directive on patients’ rights in cross-border healthcare.
Data Hub
Expands the EJP RD Virtual Platform into a federated, FAIR ecosystem that links registries, biobanks, omics resources and real-world data.
It also offers secure tools for data capture, curation, analysis and discovery, serving researchers, clinicians and regulators.
Finally, it acts as the information backbone for the CRN, the JTC projects and the Acceleration Hub.
Expertise Services
- Provides on-demand advice on biostatistics, trial design, regulatory science, health-technology assessment and ethics.
- Runs mentoring and consultancy schemes that accompany funded projects from proposal to publication.
- Works closely with the DSH to standardise methods and with the Education Hub to share good practice.
ERDERA Accelerator
- Helps mature promising diagnostics and therapies, linking academic teams with industry, investors and incubators.
- Supports intellectual-property management, market analysis and early dialogue with regulators.
- Relies on the DSH for evidence packages and on the ESH for regulatory guidance.
Training & Education
- Delivers an accredited, tiered training programme (online and face-to-face) for researchers, clinicians, regulators and patient representatives.
- Updates modules regularly to reflect lessons learnt in the other pillars.
- Ensures that everyone, from early-career scientists to experienced patient advocates, can participate effectively.
International Alignment
Through existing and newly established National Mirror Groups, the partnership ensures alignment between national and international rare disease research strategies, particularly in nations that are behind in developing and implementing national plans.
ERDERA also hosts the Scientific Secretariat of the International Rare Disease Research Consortium (IRDiRC), a unique consortium globally, co-established by the European Commission and US National Institutes of Health back in 2011.
Read how these pillars translate into concrete objectives and milestones in our Strategic Research and Innovation Agenda (SRIA).
Patient and Public Involvement & Engagement (PPIE) – at the core
People living with a rare disease and their representatives co-design priorities, sit on decision-making boards and help evaluate funded projects. Training and mentoring opportunities enable meaningful participation across the entire ERDERA structure.
ERDERA governance
The governance of ERDERA is built on collaboration, transparency and strategic coordination, ensuring that the partnership remains impactful, efficient and aligned with European and national priorities. ERDERA’s structure brings together national and international experts, funders, and stakeholders through a set of key bodies with defined roles. Discover ERDERA’s team.
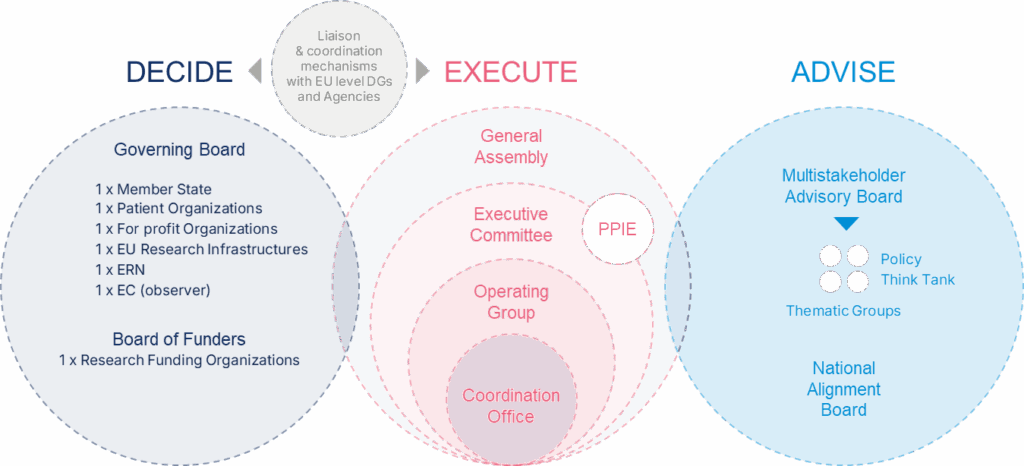
DECIDE
Governing Board (GB): The Governing Board is ERDERA’s main decision-making body. It is composed of representatives from participating countries, patient organisations, industry, EU research infrastructures, the European Commission, and other key stakeholder groups. The GB is responsible for steering the overall strategic direction of ERDERA, approving annual work plans, and ensuring alignment with European and national policies. It also plays a key role in supporting the uptake of ERDERA outcomes and planning for sustainability.
Board of Funders (BoF): The Board of Funders works alongside the GB but focuses specifically on decisions related to joint transnational calls. It ensures financial coordination and commitment from national and European funder
EXECUTE
General Assembly (GA): The General Assembly involves all partners and plays a key role in shaping ERDERA’s scientific priorities. It prepares strategic proposals—such as updates to the Strategic Research and Innovation Agenda (SRIA), work plans, and sustainability plans—for discussion and approval by the GB.
Executive Committee (ExCom): The Executive Committee oversees the day-to-day implementation of ERDERA’s activities. Composed of the Coordination Team, Work Stream leaders and Work Package leaders, it ensures progress towards milestones, quality control of outputs, and alignment with the strategic plan. It prepares content for decision-making by the GB and handles any necessary adjustments during implementation.
Operating Group (OG): The Operating Group includes the Coordination Team and Work Stream leaders. It ensures smooth internal communication and coordination across work streams, supporting reflection on ERDERA’s progress and future directions.
Coordination Office: Led by INSERM, the Coordination Office manages the scientific, administrative, and financial operations of the partnership. It acts as the main liaison with the European Commission and represents ERDERA at both EU and international levels.
ADVISE
Multistakeholder Advisory Board (MAB): The MAB consists of 20–25 independent experts from various sectors, including patient organisations, academia, regulatory bodies (e.g., EMA, FDA), European Reference Networks (ERNs), HTA representatives, and ethicists. Its role is to provide strategic advice and help shape the partnership’s research and support activities. MAB ensures that patient needs are central, and it can establish Thematic Groups to expand expertise when needed.
National Alignment Board (NAB): The National Alignment Board brings together representatives from each participating country’s National Mirror Group and, where relevant, the European Commission. It supports national alignment and exchange of best practices and includes voices from underrepresented countries. It may also liaise with broader European networks to maximise impact.
